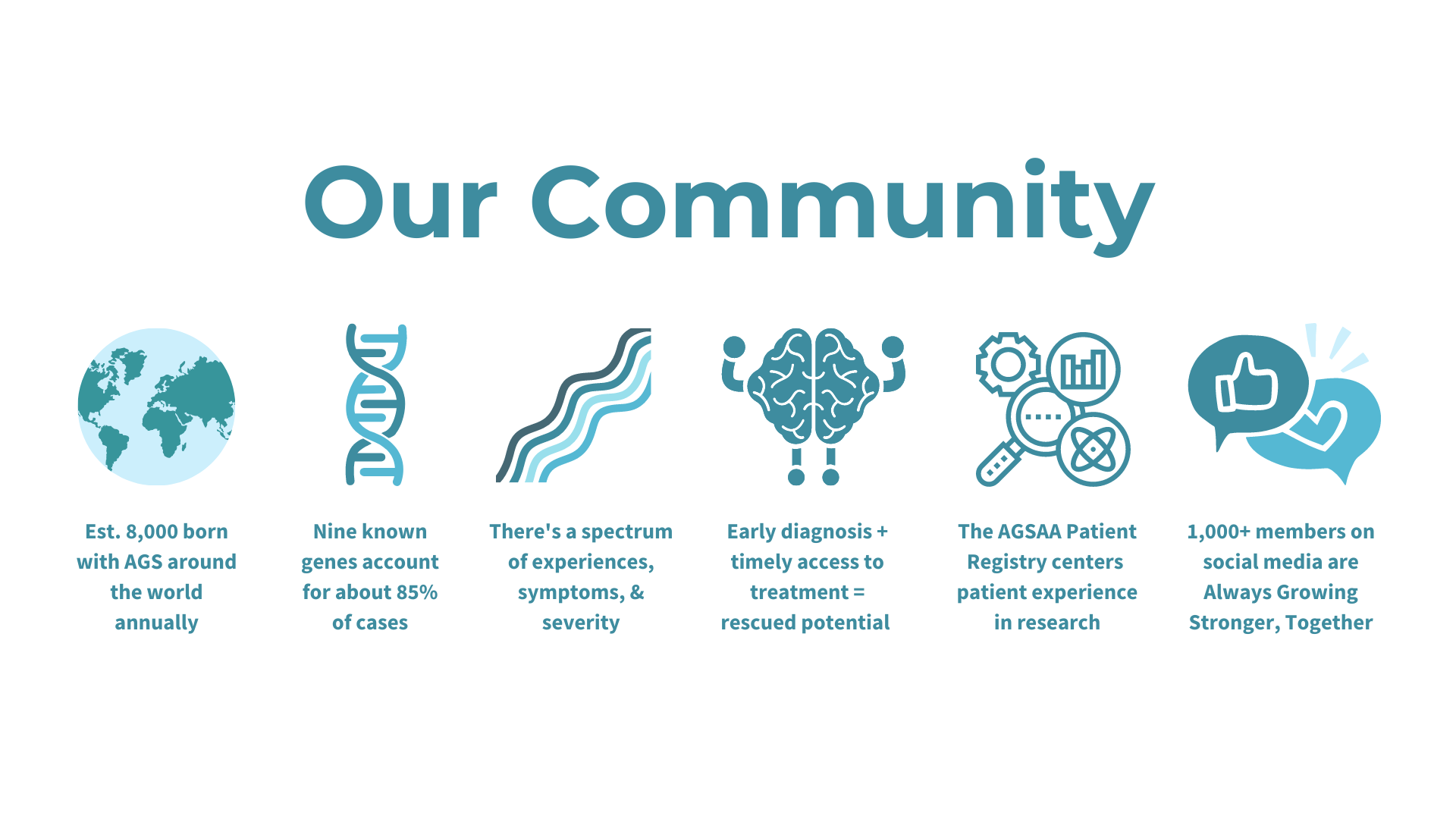
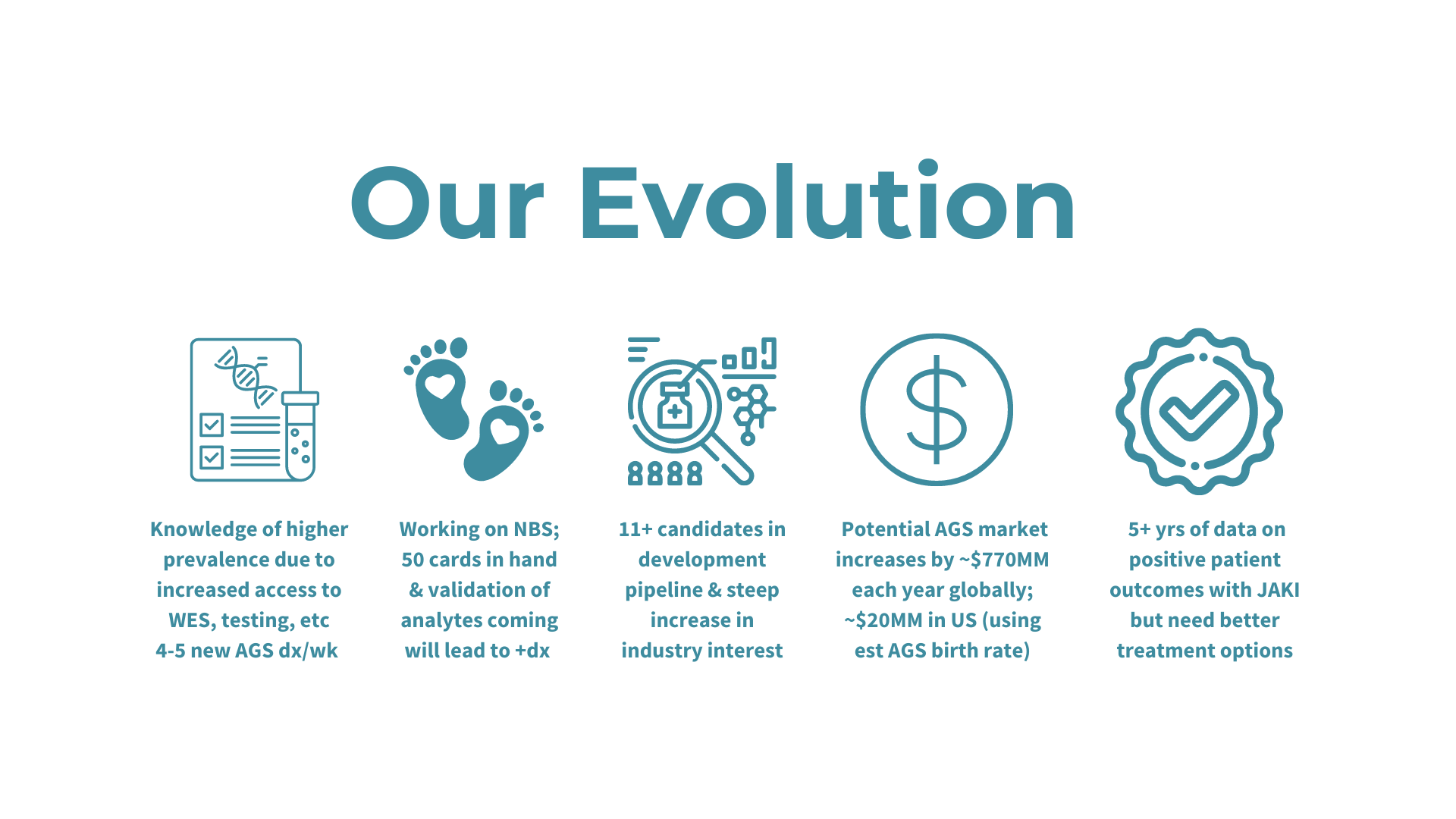
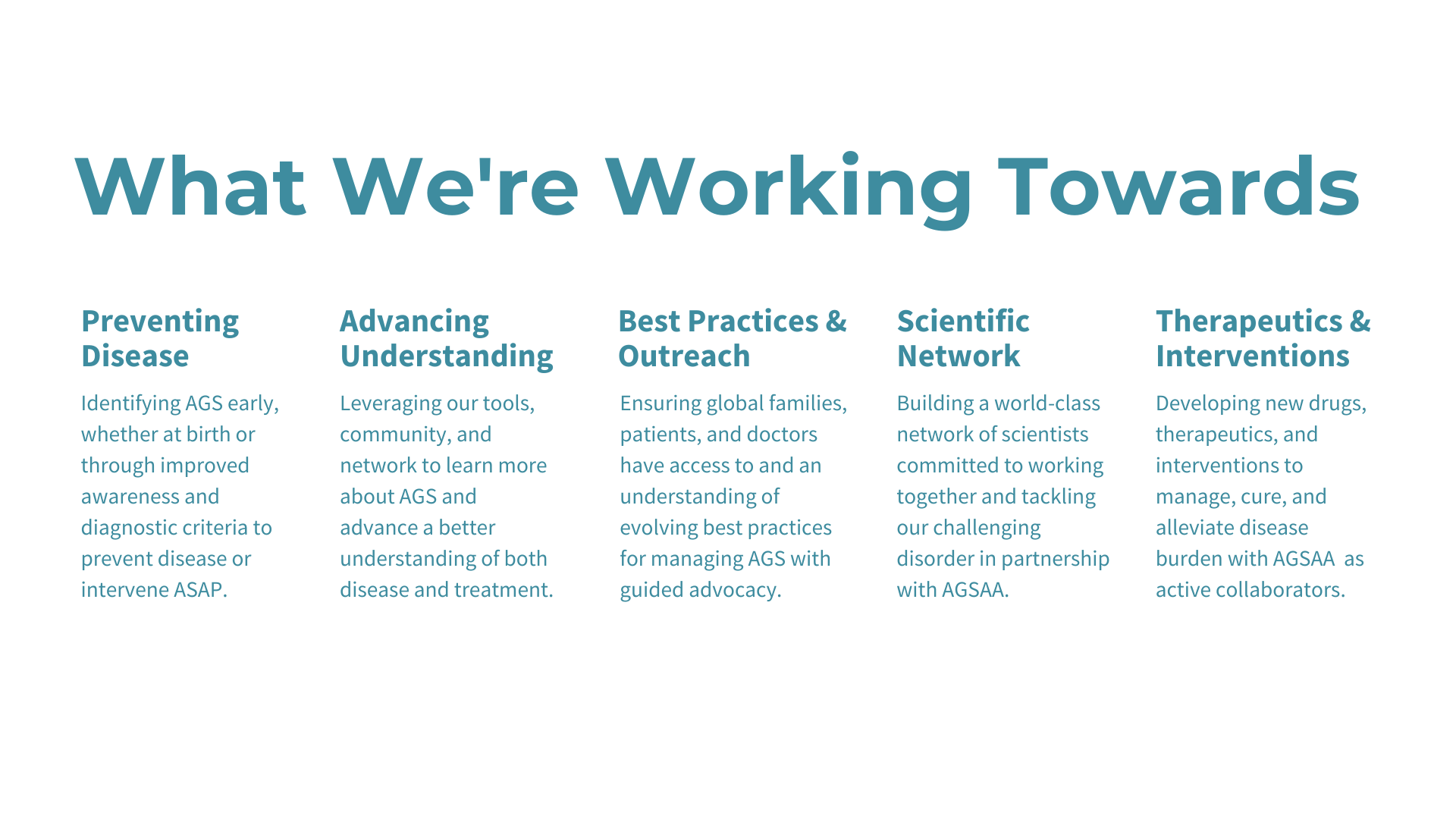
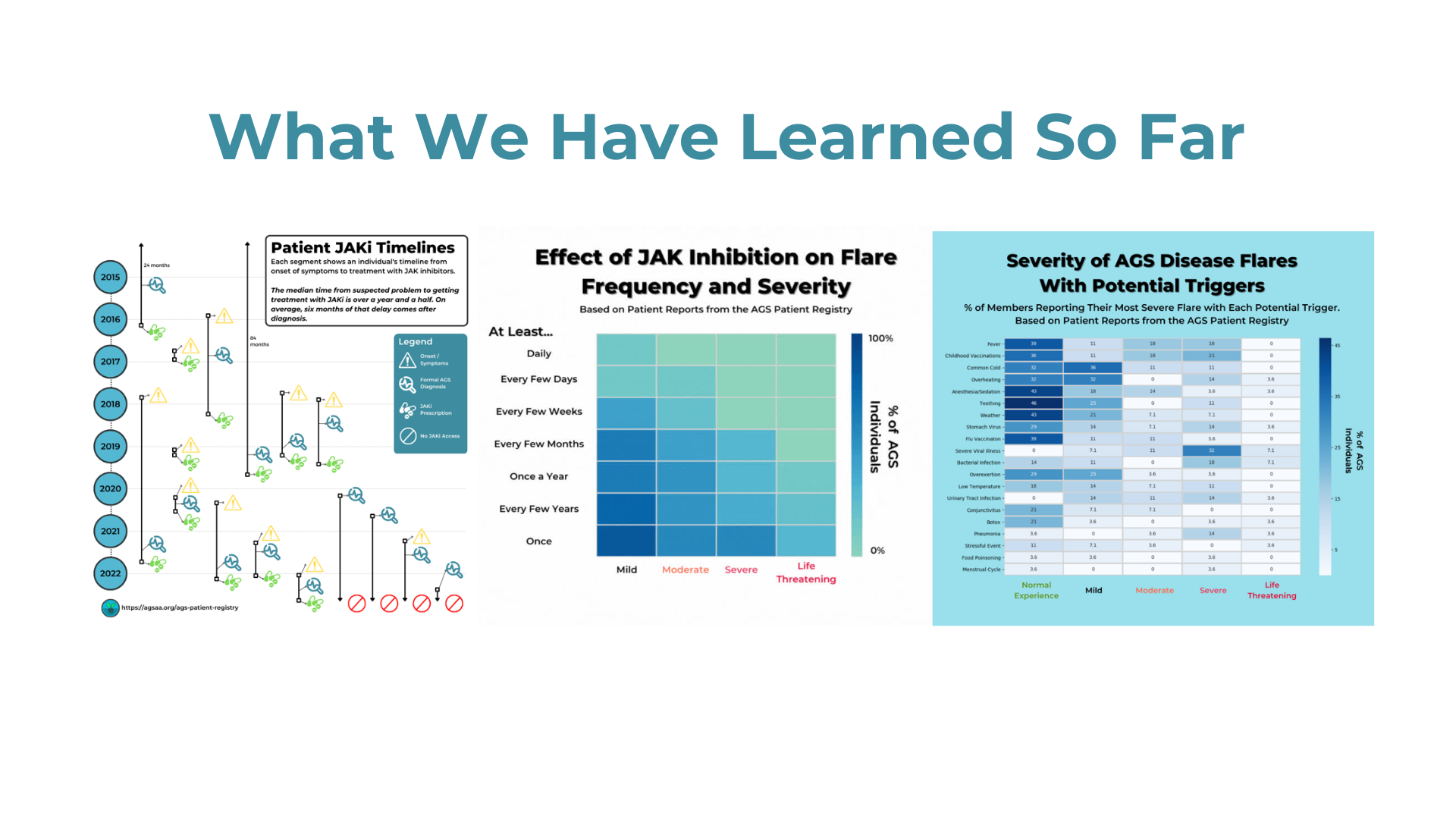
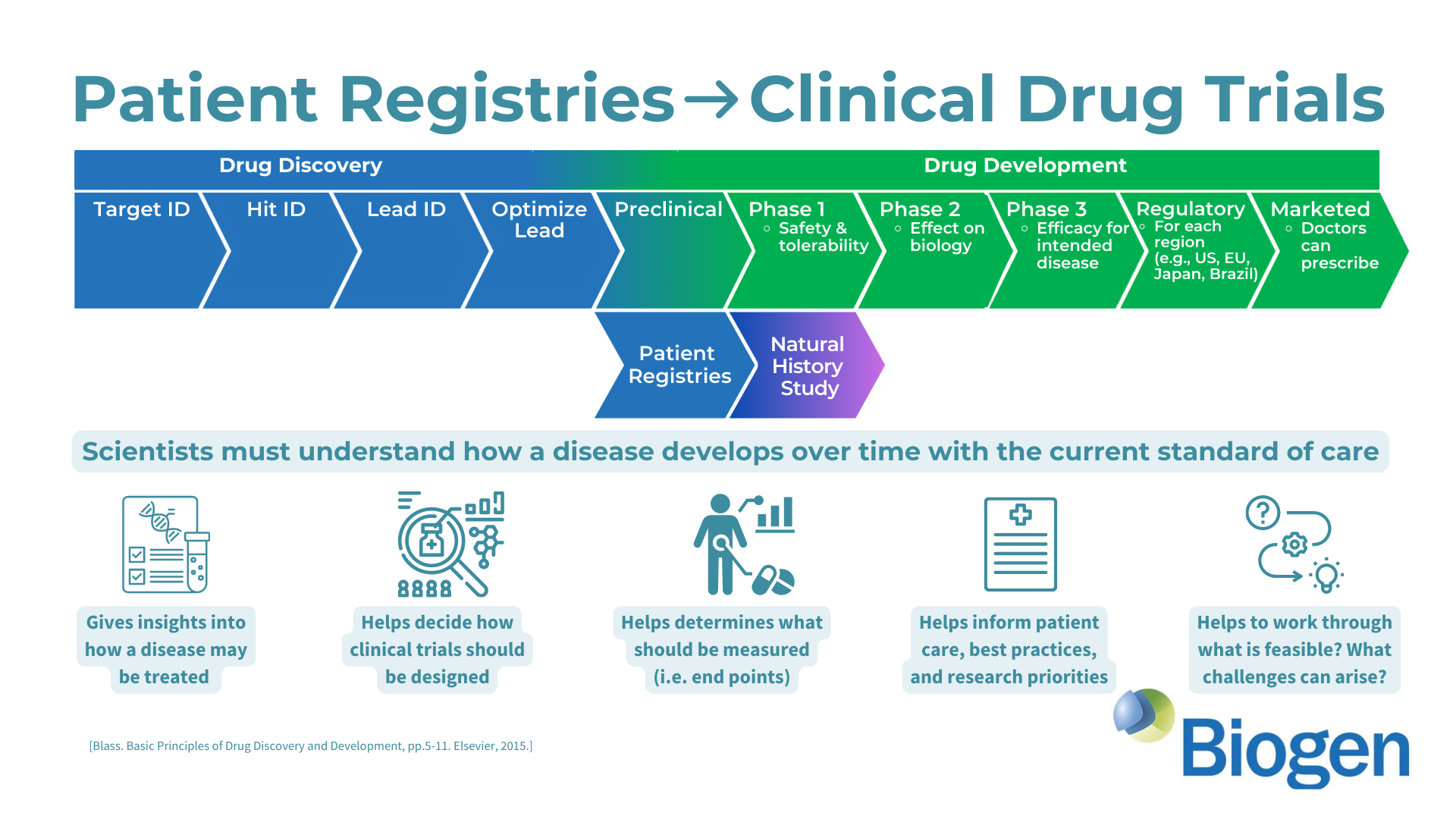
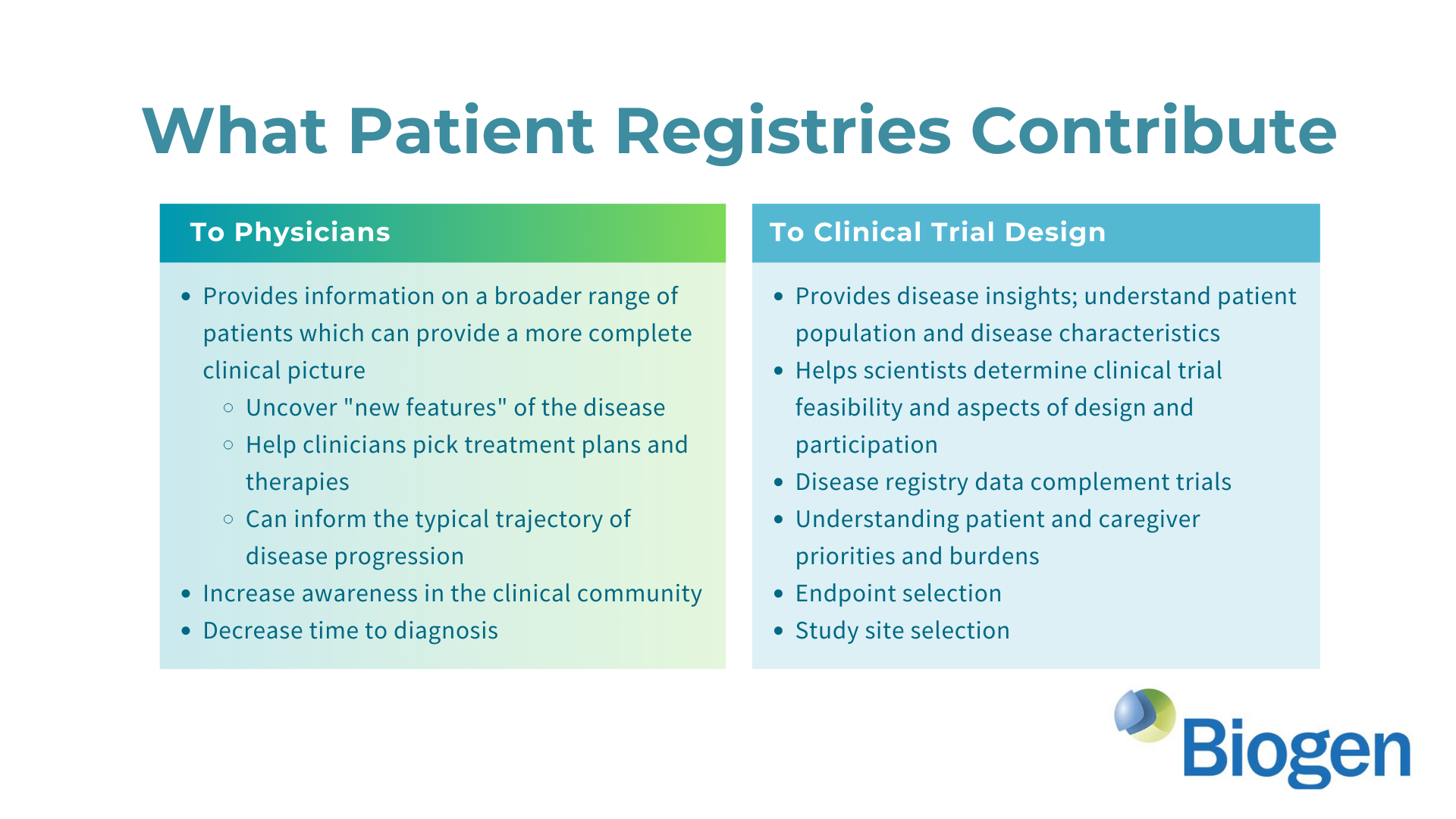
Researchers from the University of Sydney have announced a breakthrough in the treatment of Aicardi-Goutières Syndrome (AGS). Published in The Journal of Clinical Investigation, their study introduces a promising RNA-targeted therapy that effectively halts the progression of AGS in mice. The therapy targets the interferon alpha receptor using antisense oligonucleotides, reducing neuroinflammation and neuronal damage while restoring the integrity of the blood-brain barrier. The findings offer hope for potential reversal of AGS's detrimental effects and lay the groundwork for future human trials.
The study represents a significant advancement in AGS research and treatment. Lead author Dr. Barney Viengkhou and Research Director Patrick Winters of the Aicardi-Goutières Syndrome Advocacy Association express enthusiasm for the therapy's potential to alleviate the burden on affected families. Conducted in collaboration with Ionis Pharmaceuticals and Biogen, the research not only promises to transform the lives of children with AGS but also offers hope for therapy development in other childhood dementias, highlighting the urgent need for innovative treatments and support for affected families.
Read the press release from the University of Sydney:
New therapy shows promise for a rare childhood dementia. 18 April 2024
Read the paper from the Journal of Clinical Investigation:
Viengkhou, Barney et al. “Interferon-α receptor antisense oligonucleotides reduce neuroinflammation and neuropathology in a mouse model of cerebral interferonopathy.” The Journal of clinical investigation vol. 134,4 e169562. 9 Jan. 2024, doi:10.1172/JCI169562