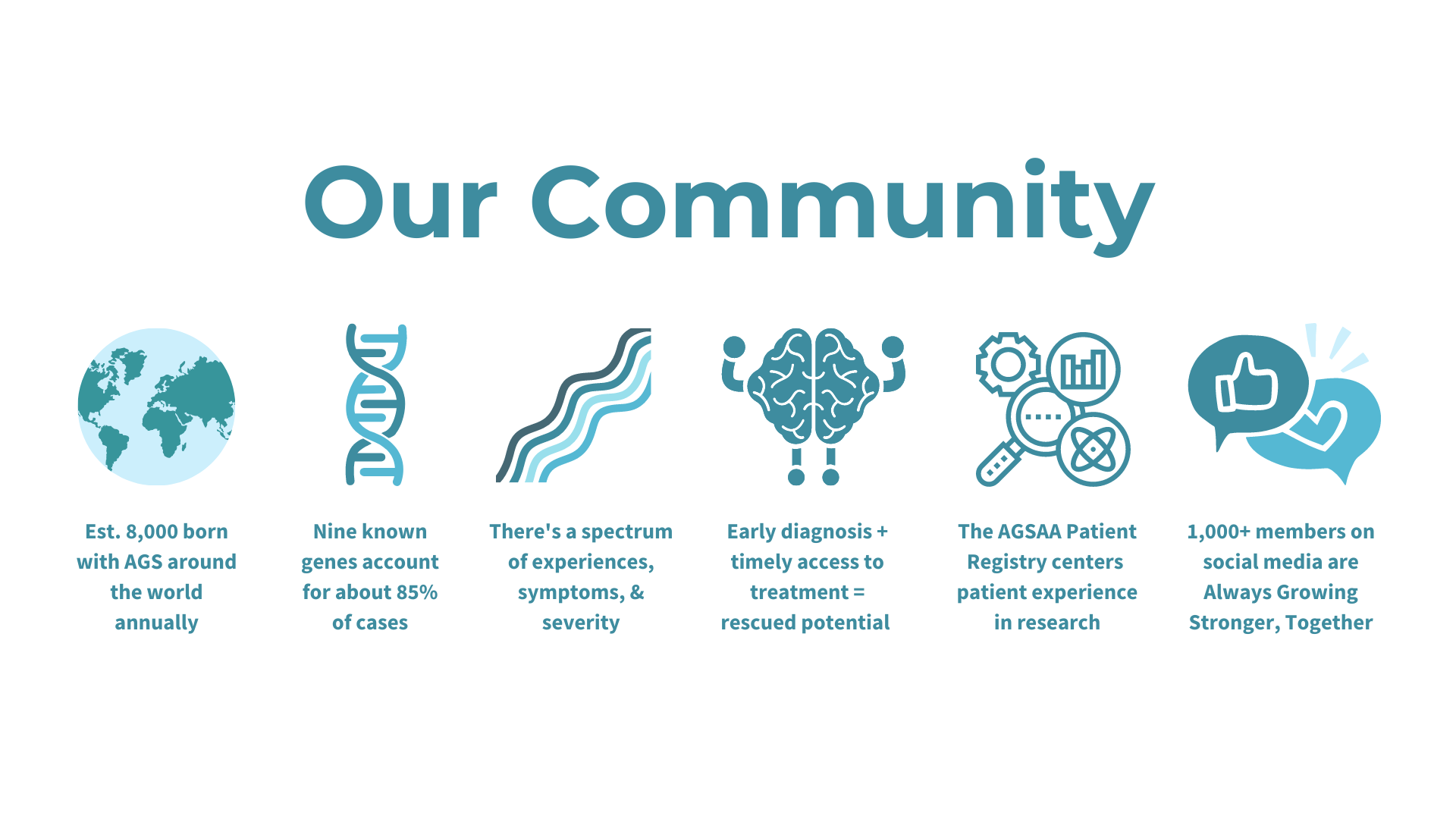
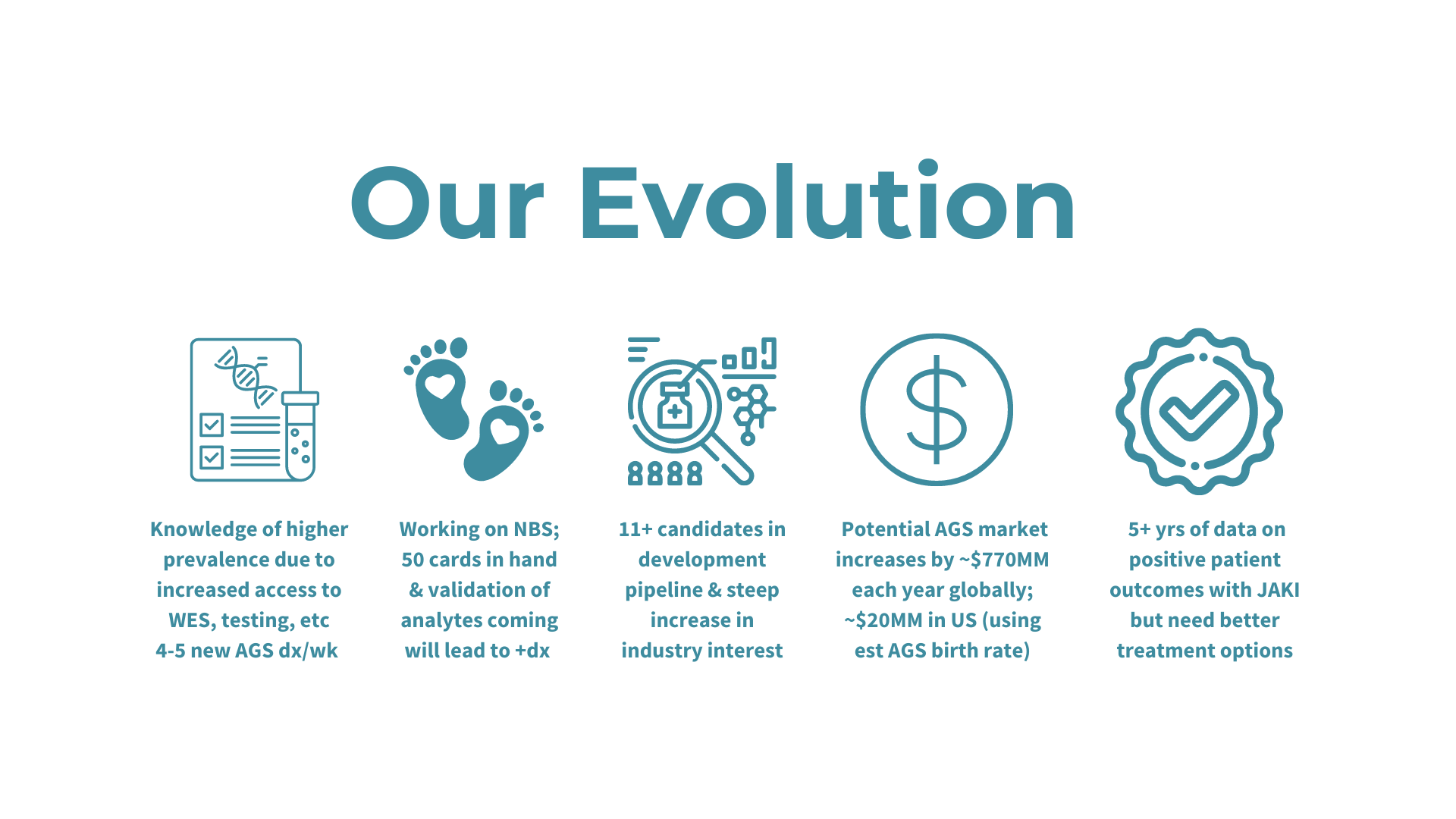
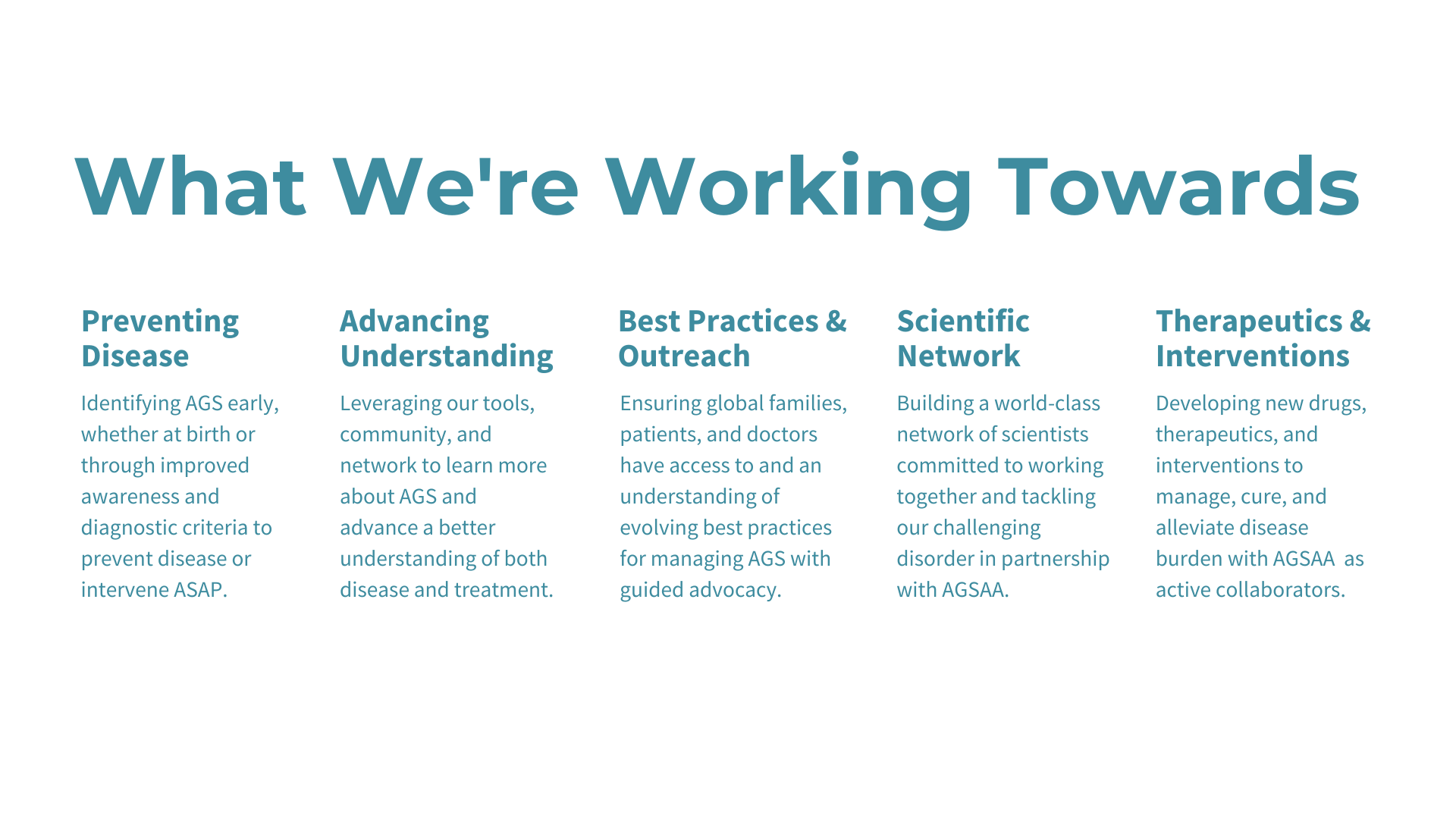
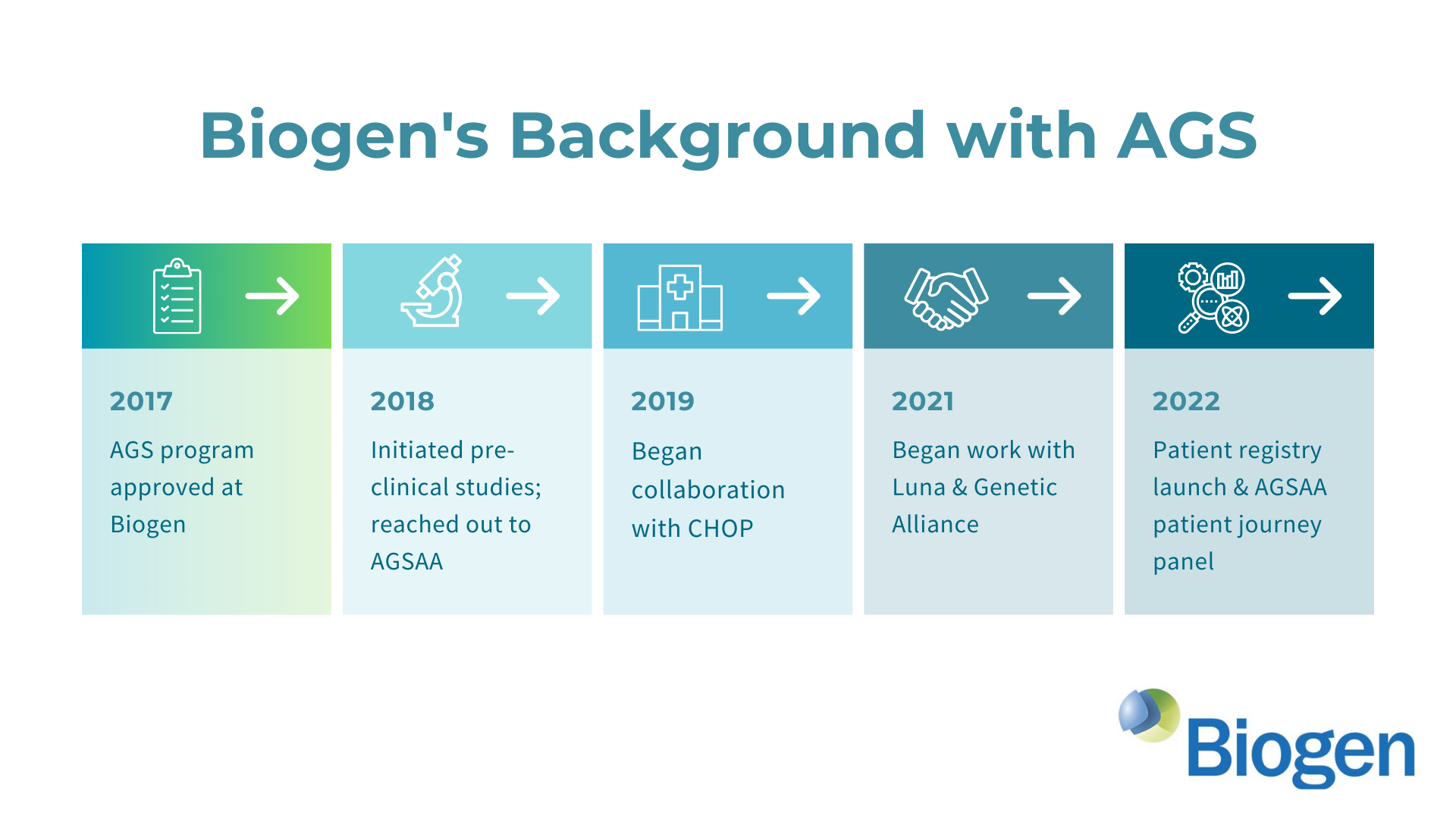
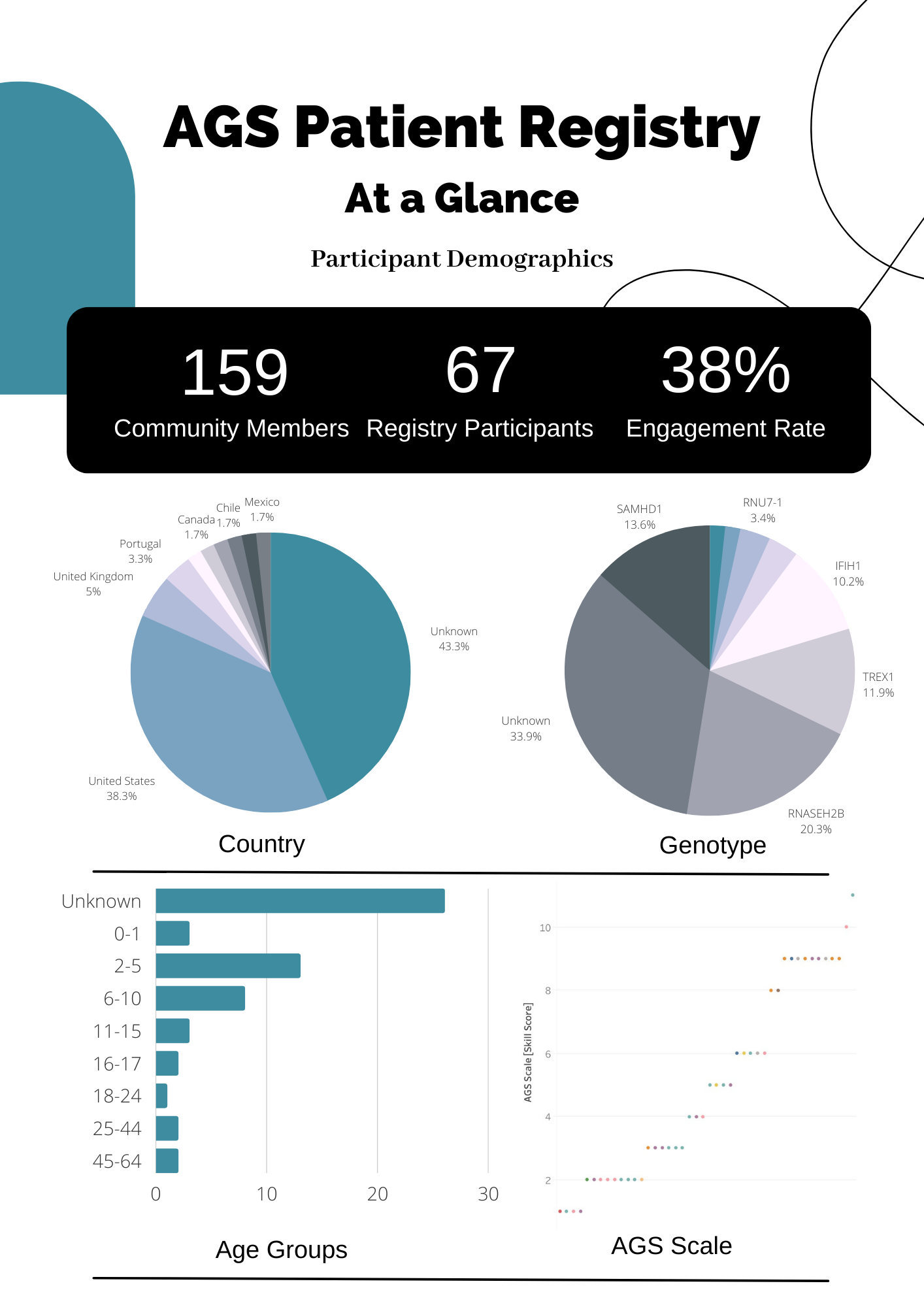
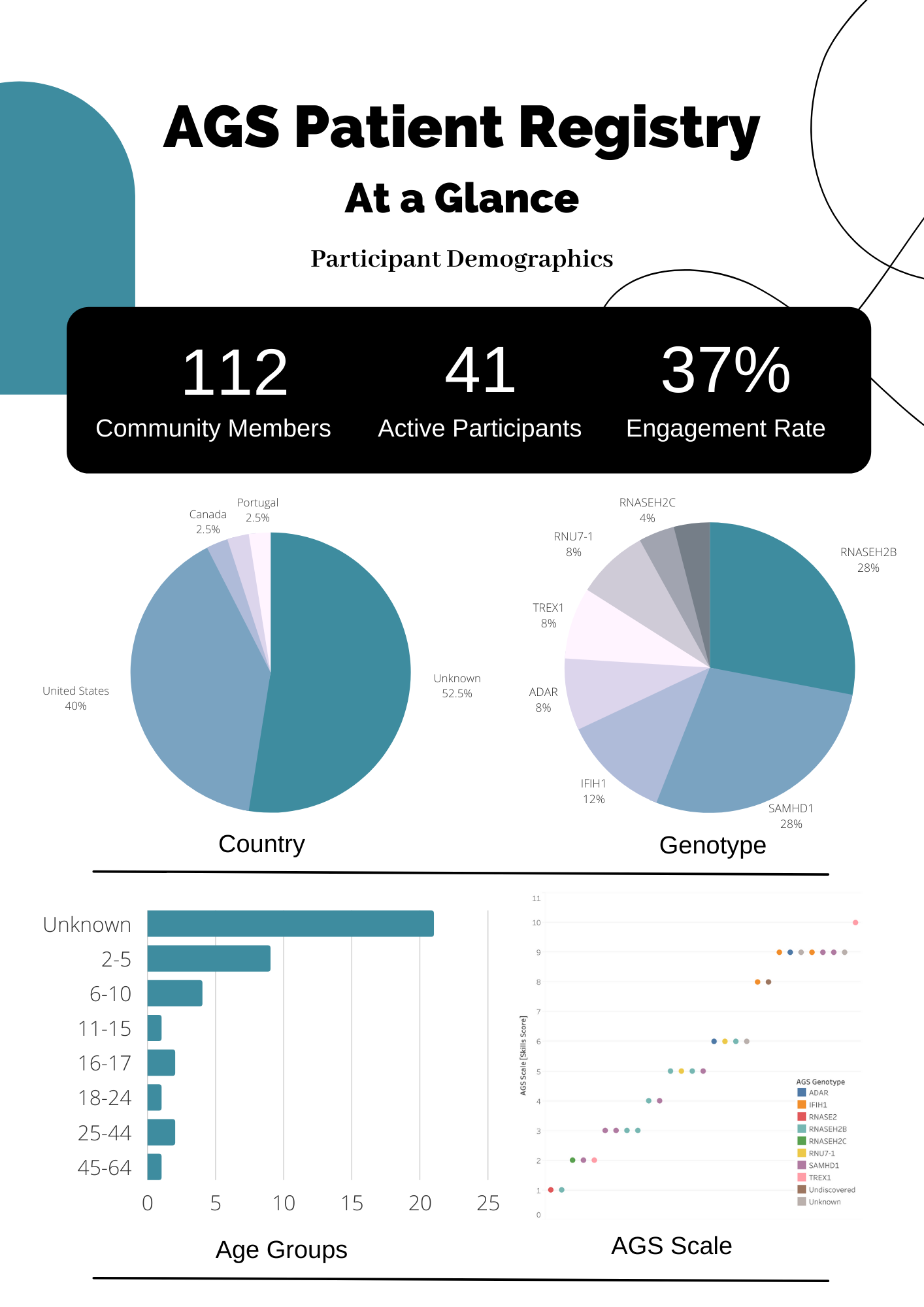
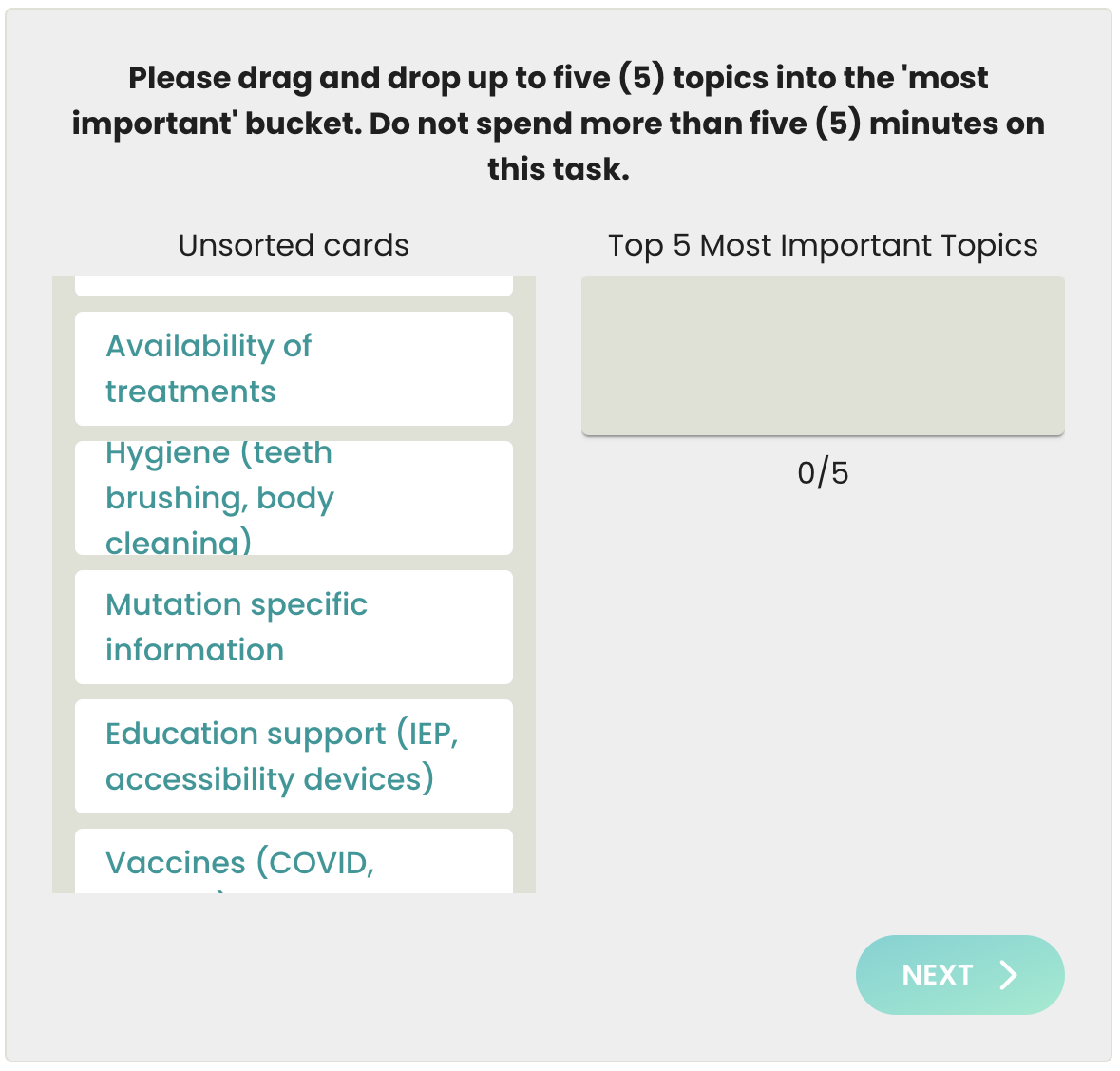
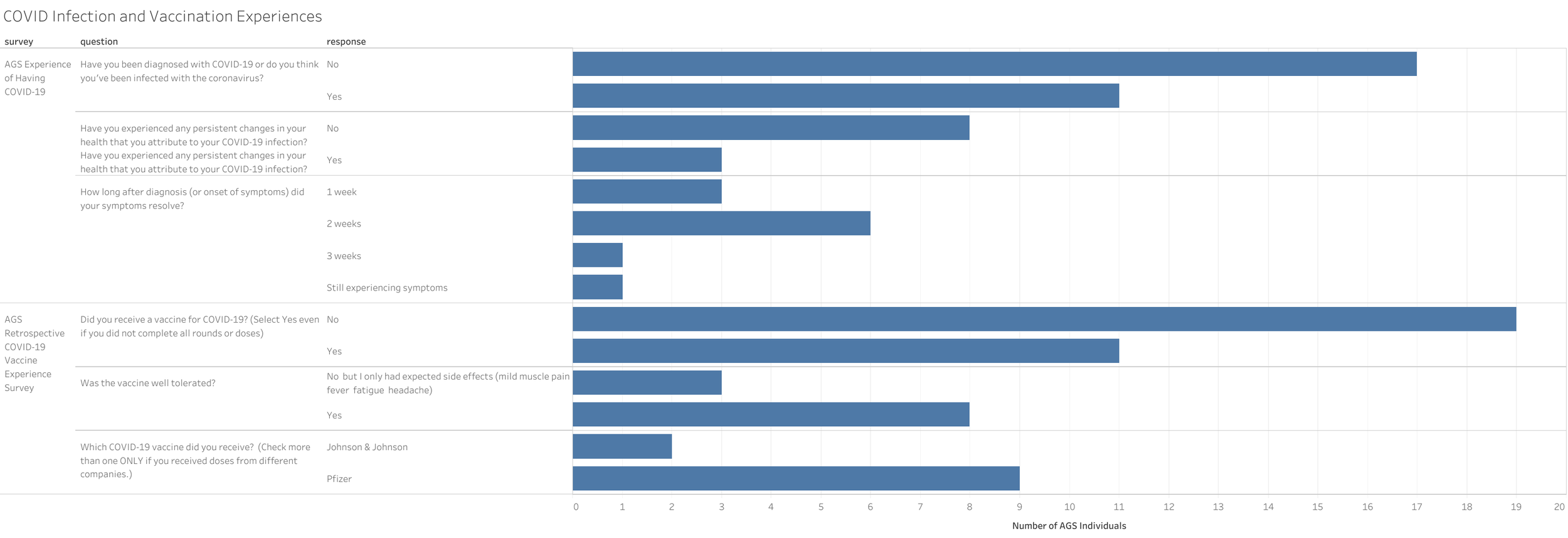
Update: Our patient registry project with Biogen has been completed as of January 2024 and we are now pivoting to our AGSAA Contact Registry and a partnership with Ciitizen to create an AGS Natural History Study by using patient medical records to unlock key insights and data for research and treatment opportunities - both launching April/May 2024.
AGS Families,
We’ve had a LOT going on this year end but wanted to quickly share some great and time-sensitive news - Biogen & AGSAA have just re-opened a unique opportunity for you to make a meaningful and direct impact on the development of an upcoming AGS clinical trial!
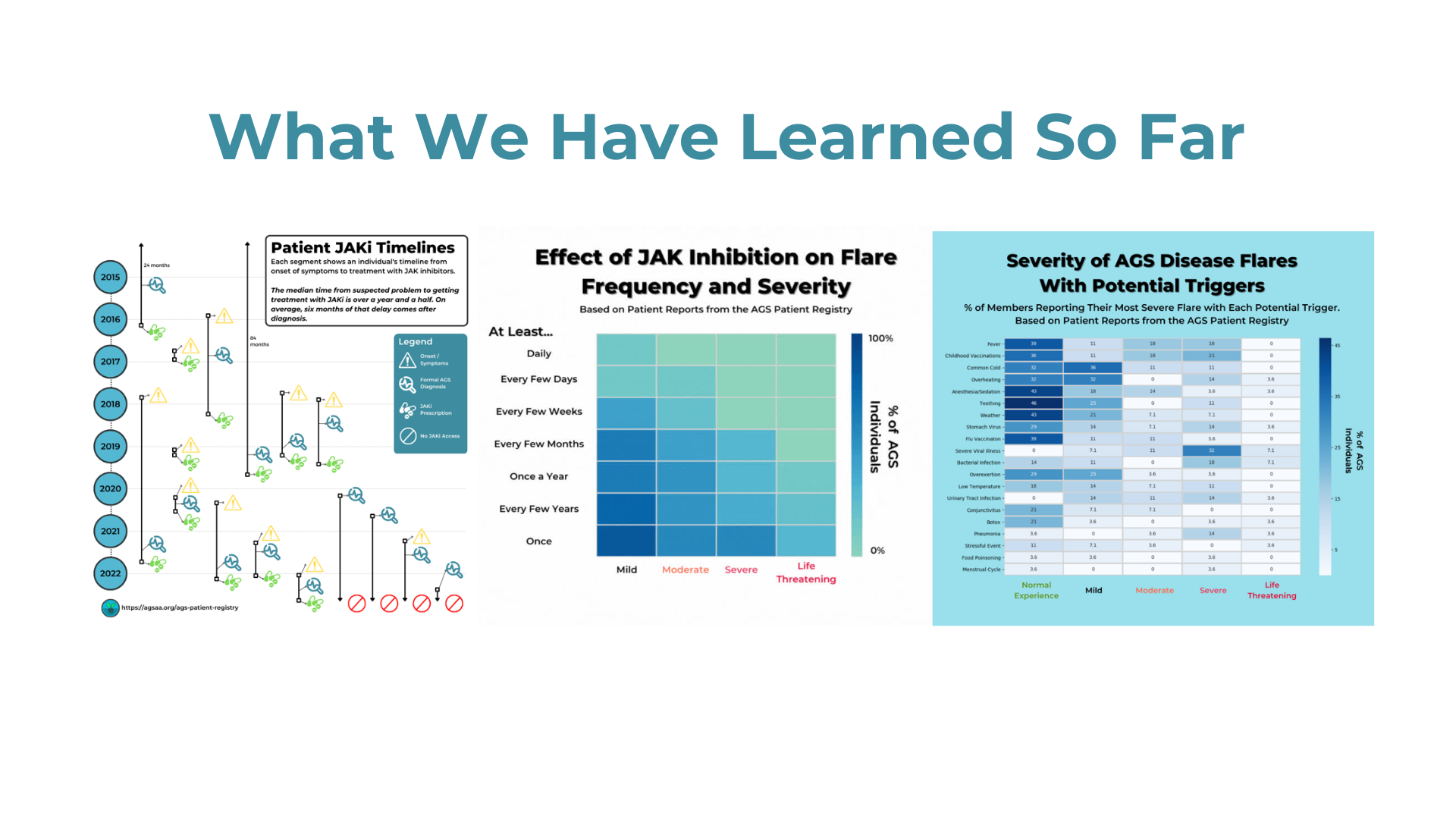
We want to continue to collect as much community driven insight and data as possible in advance of key planning meetings taking place in January. If you haven’t already shared your perspective, please take less than 30 minutes to participate in the patient registry study sponsored by Biogen aimed at understanding AGS better and improving the clinical trial development process!
💡 Why Participate?
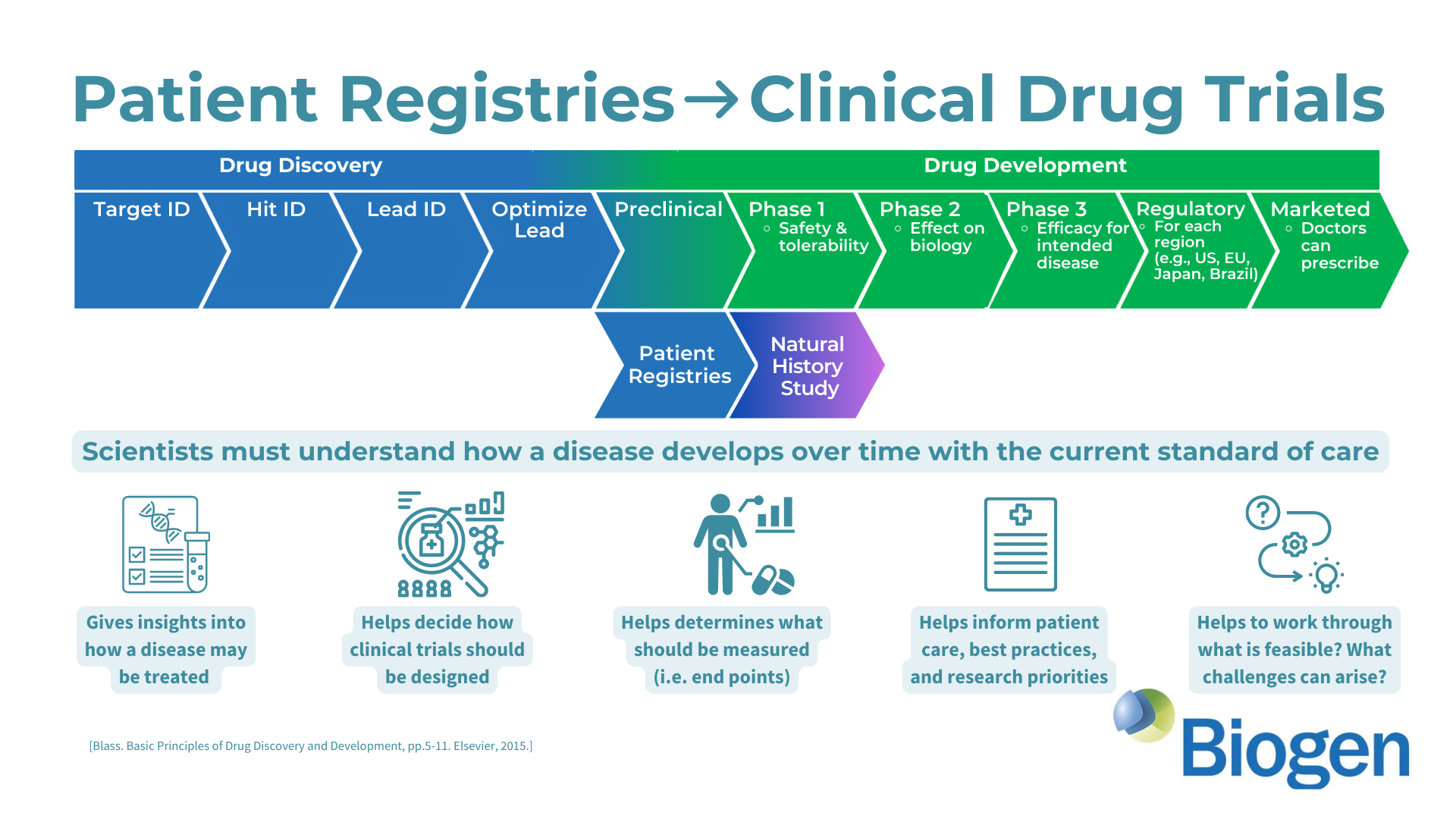
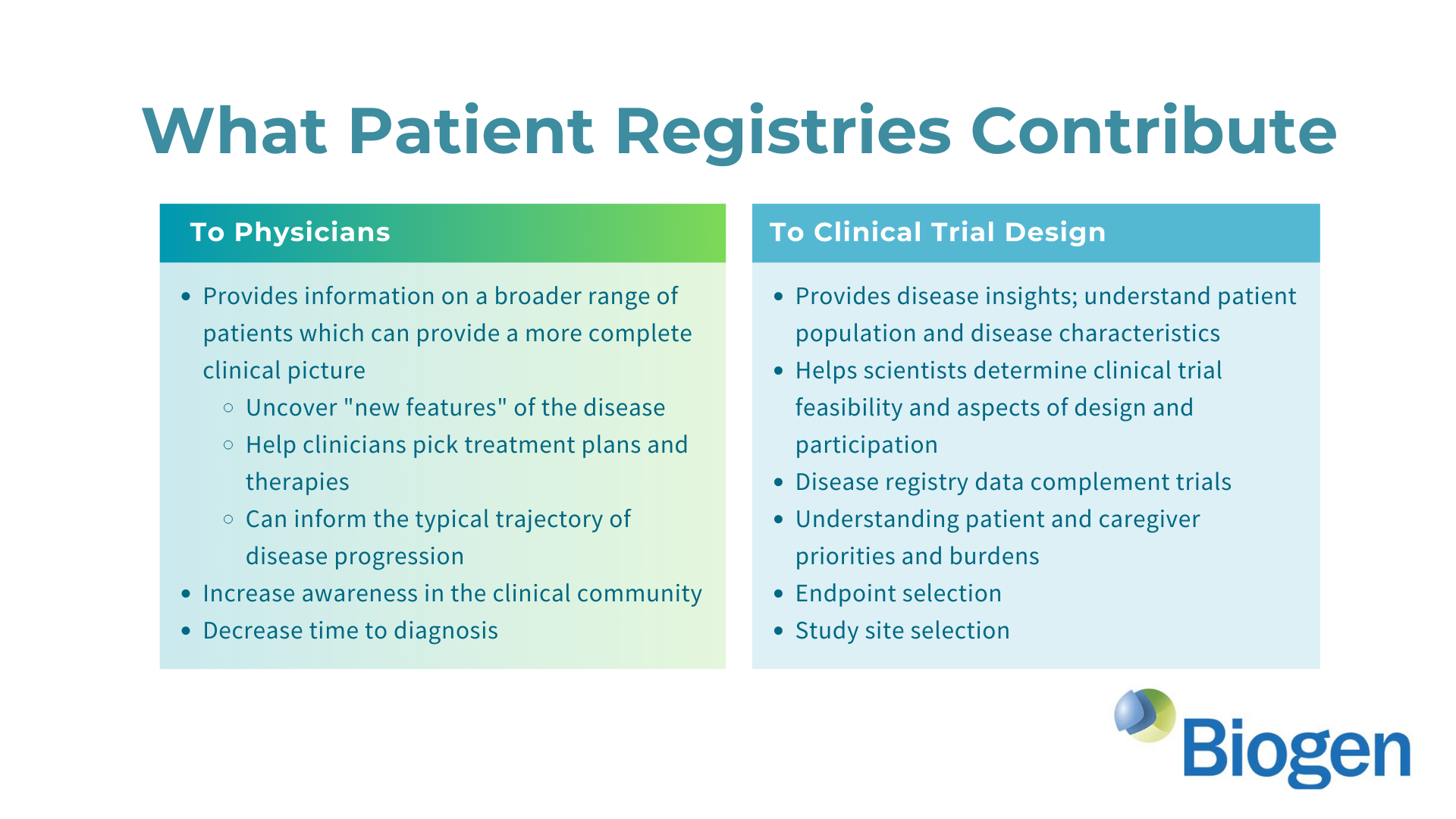
Make a Difference: Your valuable insights will contribute to advancing a Biogen clinical trial in AGS and potentially lead to a more effective and convenient clinical trial.
$50 Amazon Gift Card: As a token of appreciation for your time, Biogen is offering a $50 Amazon gift card to everyone who completes the survey.
30 Minutes Well Spent: The survey will only take about 30 minutes of your time, but the impact it can have on AGS research is immeasurable!
👉 How to Participate:
Click on the survey button below to get started.
Complete the survey with your honest responses.
NEW: For security purposes, you will also be asked to briefly record a few responses so we know for sure that you are bringing your own AGS impacted life experiences to this survey.
Share this announcement with other AGS families to maximize our collective impact.
🌐 Learn More:
To delve deeper into the world of AGS research and clinical trial development, consider these resources:
AGS Patient Registry: Stay connected with the AGS community and access valuable information. Link to AGS Patient Registry.
Biogen Webinar: Discover more about Biogen's commitment to AGS research in our first webinar and learn why this is all so important. Link to the Webinar.
Let's unite as a community and contribute to a brighter future for those with AGS. Your involvement is significant, and together, we can make a real difference.
Thank you for being the incredible AGS advocates that you are. Let's spread the word and make AGS history together!